On the Front Lines of the COVID-19 Crisis

Leading with Purpose means persevering in the face of hardship and adversity. And Parker team members are doing exactly that as they work to solve the unique challenges presented by the coronavirus pandemic.
Purpose in Action on the Front Lines of the COVID-19 Crisis
One recent example is the collaboration between Parker's Engine Mobile Aftermarket (EMAM) Division and HVAC Division to provide a new government-approved HVAC filtration solution to up to 8 remote hospital sites in New Jersey—one of several regions in the U.S. where patient intake has greatly outpaced the capacity of local hospitals.
While the two divisions have partnered many times in the past, this project was anything but ordinary. The team needed to develop a filtration solution for an air handling system used to generate clean air for emergency workers in medical tents set up for overflow care. And they needed to act quickly.
"We went from concept to delivery in five days," explains Steve Zimmerman, Division Marketing Manager for the Engine Mobile Aftermarket Division. "A longtime EMAM customer came to us for a solution and both divisions immediately got to work. The entire team understood both the critical importance of the application and the urgency of the situation."
The EMAM and HVAC divisions took special precautions and ramped up production to safely deliver on this order. And the need for these types of solutions continues to increase.
"When there's a direct correlation between the products we make and a global problem that needs solved, our team goes above and beyond to get the job done," says Zimmerman. "We're more than willing to put the greater purpose ahead of our own."
Ramping Up Ventilator Enabling Technologies
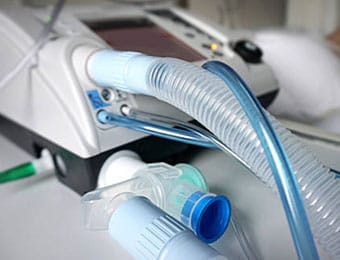
Parker's Precision Fluidics Division is expanding production in response to significantly increased demand for solenoid and proportional valves used in critical ventilation and patient monitoring equipment needed to treat patients with COVID-19.
Ramping Up Ventilator-Enabling Technologies for Pandemic Relief Effort
With 20+ years of experience serving the medical device industry, the division is well positioned to respond to customer needs with technical expertise and lean operations to ensure product quality and reliability.
Parker's miniature solenoid valves are known for high-speed operation and extremely low leak rates for use in medical devices, while its proportional valve technology meets the most demanding precision flow applications in the industry. Most of its valves can be cleaned for Oxygen use per ISO15001:2010 and many also meet ISO10993 Biocompatibility requirements.
Several valves from Precision Fluidics are used to support ventilator and patient monitoring systems including the VSO Max HP, LM-Pro, VSO, VSO Low Pro, x-Valve, V2 and S-11 valves.
Existing relationships with customers who serve several key global ventilator and patient monitoring manufacturers have led to a successful expansion of the division's operations to meet increasingly demanding production requests.
A global leader in ventilator instruments stated the following: "We're very moved by Parker Hannifin employees' strong support on the prevention of the coronavirus, which is worthy of our admiration and praise. Our company relies on socially responsible suppliers like Parker Hannifin, and we expect your company continue to support us on production and jointly contribute to the coronavirus prevention."
"We are taking many preventative measures locally to address our highest priority, which is the safety of our team members, their families and our community, while addressing the critical demand of our customers," said Craig Stiffler, Precision Fluidics General Manager.
The division's team based in New Hampshire are all stepping up by adopting rotating schedules and additional shifts, helping to onboard new hires and moving from the office to manufacturing cells to support the increased production.
The agility of the local team is a key factor in their ability to quickly adjust to rising demands in this time of crisis. The division has increased capacity across three primary manufacturing cells to meet the unprecedented increased demand for critical valves that have the potential to save lives.
"Our entire team has responded with the courage and determination that we are all known for at Parker," continued Stiffler. "I'd like to extend my thanks to each of them for adhering to our enhanced hygiene and disinfection protocols and implementing physical distancing practices, to ensure we can continue to supply our critical valves to help treat sick patients."
EMDN Supports Critical COVID-19 Testing
Laboratories and point-of-care testing operations are processing unprecedented numbers of COVID-19 tests to ensure proper diagnosis of individuals and care for those who are sick.
Electromechanical Division Supports Critical COVID-19 Testing, Vaccine Development and their Community
Parker's Electromechanical & Drives Division - North America (EMDN), with locations in California, Ohio, Minnesota, North Carolina and Pennsylvania, supplies precision actuators and servo motors to enable genomic sequencing, drug discovery, and high throughput testing.
As the need for medical and scientific equipment began to accelerate, key customers reached out to EMDN to request expedited orders and backlog deliveries as soon as possible to support different aspects of COVID-19 testing, treatment and prevention.
Two key products from the division are used to support precise, multi-axis system configurations that ensure excellent repeatability and reproducibility in automated processes.
The 400XR Series of precision linear mechanics is recognized globally for its consistent performance, high quality, high strength- yet sleek design, and unparalleled versatility. The mSR 100 is a miniature, high precision linear positioner with an extremely compact design. These technologies are often used in precise sampling operations to support tables with multiple vials processed simultaneously.
One customer was a pioneer in the testing process for COVID-19 and is FDA certified to produce point-of-care testing equipment. Beginning in April, they are targeting the production of 600,000 tests per month.
A second customer uses Parker's precision actuators to focus on genome sequencing to inspect samples to support the development of a potential vaccine for COVID-19. Understanding the epidemiology of the virus has helped to inform transmissibility, virulence (how dangerous the virus is to individual patient populations), and the variation of targets for therapy.
Relatedly, another Parker customer specializing in the development of a COVID-19 vaccine, is using our precision motors to spin asset trays that examine the effectiveness of different treatments. They have also secured FDA approval to produce swabs, vials and prepackaged testing kits.
In addition, the division is working closely to support increased production of machines that will manufacture mass quantities of vials to support vaccine distribution.
"The whole team has stepped up to prioritize critical orders from these important customers," said Chris Griffin, EMDN General Manager.
Across the division's five locations in the U.S. there are about 375 team members who are dedicated to producing these essential actuators and motors to support the important work being done to stop COVID-19.
EMDN team members are also teaming up to support those in need within their community with a recent donation to Second Harvest Food Bank of Metrolina to help feed those impacted by COVID-19. The Food Bank is working with over 800 partner agencies in 24 counties, including the local school systems, to provide healthy shelf-stable items for food boxes. These boxes will help feed families whose children are missing school meals, seniors being asked to stay safely in their homes, those in need of food who are quarantined, and team members in our community being impacted by decreases in work hours.
"The rapid development of a vaccine for COVID-19 is so important in putting an end to this devastating, global health crisis," noted Griffin. "The dedication of our team members to support the community and follow safety protocols while providing excellent manufacturing support and delivering urgent orders during such a difficult time has been remarkable. I'd like to thank everyone who continues to support this important effort."
