Medical Systems
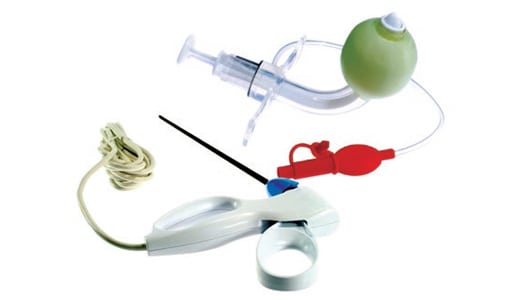
Parker's Medical Systems Division is a global, FDA registered, ISO 13485 certified, clean room manufacturer of custom medical components and assemblies for the medical, pharmaceutical and life science industries.
With extensive prototype and production tooling capabilities, the Medical Systems Division offers medical and pharmaceutical OEMs a wide range of medical grade contract-molding services by way of liquid silicone injection molding (LIM/LSR), silicone injection & flash-less-molding, organic rubber injection & flash-less molding, and compression molding. The division also offers extensive thermoplastic and TPE/TPU injection molding at its six manufacturing facilities in California and Indiana. It manufactures medical grade silicone tubing, medical device assembly to individual OEMs specifications.
Parker's Medical Systems Division uses tested and certified medical and implantable grade silicone, thermoplastic and TPE base materials from key material suppliers for molding elastomeric medical components. It offers its customers customized material formulations whenever necessary per the OEMs specific functional requirements.
Visit the Medical Systems Division Website
Medical Systems Division
(Headquarters)
3191 E La Palma Avenue
Anaheim, CA 92806
Ph: 714-632-7710
Fax 714-632-5647